Abstract
Introduction: Between 50-80% of patients with diffuse large B-cell lymphoma (DLBCL) are cured by frontline (1L) R-CHOP immunochemotherapy. Ultra-high risk (UHR) features for poor overall survival (OS) include: progression through the frontline therapy (primary progression, PP), presence of a MYC translocation (MYC-R+), and a high or high-intermediate National Comprehensive Cancer Network International Prognostic Index (NCCN-IPI) (Costa, Am. J. Hematol., 2017). We aim to explore the role of these UHR factors in the outcomes of DLBCL patients receiving standard of care (SOC) anti-CD19 CAR T-cell therapy.
Methods: This is a retrospective single-center study of relapsed/refractory (R/R) DLBCL patients treated with either axicabtagene ciloleucel (axi-cel) or tisagenlecleucel (tisa-cel) as SOC at Moffitt Cancer Center according to the FDA label as of March 2021, or who were treated on the expanded access programs (EAP) for axi-cel (NCT03153462) and tisa-cel (NCT03601442) for the provision of CAR T when products fell outside of manufacturing specifications (OOS). We excluded patients who had received prior therapy for indolent B-cell lymphomas (iNHL). We defined patients with primary treatment failure (PTF) as: PP, residual disease after 1L therapy (RD), or early relapse within 6 months of 1L therapy (ER). For patients with PTF, we calculated the number of UHR features (0 to 3): MYC status, NCCN-IPI, and PP. Kaplan-Meier survival curves were used to compare progression free survival (PFS) and overall survival (OS) starting from the date of CAR T-cell infusion, with statistical significance determined using the log-rank test at the P<0.05 threshold.
Results: A total of 187 R/R DLBCL patients received SOC or EAP CAR T-cell therapy, of which 116 had DLBCL with no prior therapy for iNHL and were included in this analysis. PTF occurred in 75 patients (65%), of which 30 (40%) patients had primary progression as the failure pattern, 23 (30.7%) patients had MYC-R detected by FISH, and 37 (49.3%) patients had intermediate-high/high NCCN-IPI scores at the time of PTF. The median follow up was 10.05 months. Of the 75 patients with PTF, 69 received axi-cel and 6 received tisa-cel. Main 1L therapies were R-CHOP in 59 (78.6%) cases and DA-EPOCH-R in 14 (18.7%). The median lines of therapy prior to CAR T-cell therapy was 3 (range 2-6 lines).
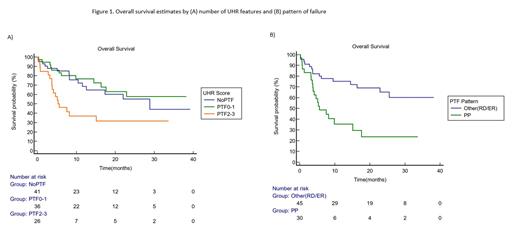
The number of UHR features was associated with a shorter OS after CAR T-cell therapy. The OS for patients with 2-3 and 0-1 UHR were 5.3 months (95% CI, 3.7 to 15.13 months) and not reached, respectively (P=0.005; Figure 1A). In terms of PTF patterns, PP was associated with worse PFS and OS after CAR T-cell therapy compared to other patterns (RD/ER) (PP, mPFS 3.1 months vs RD/ER, mPFS not reached; p<0.001; PP, median OS 5.63 months vs RD/ER, mOS not reached, P<0.001; Figure 1B). Patients with PTF and MYC-R+ had no difference in PFS (P=0.51) but a shorter OS after CAR T-cell therapy compared to those without an identified MYC translocation (P=0.05). Patients with intermediate-high or high NCCN-IPI at time of PTF had similar PFS (P=0.75) and OS (P=0.34) to patients with intermediate-low or low NCCN-IPI.
Conclusion: Patients with DLBCL who experience PP to frontline immunochemotherapy had shorter PFS and OS after subsequent CAR T-cell therapy compared to other PTF patterns. R/R DLBCL patients with PP represent a poor prognosis subgroup, even with CAR T-cell therapy. It will be important to determine if patients with primary progression have increased benefit from CAR T-cell therapy if it is provided at first relapse rather than after 2 or more prior lines of therapy. Our study suggests that mechanisms of tumor resistance to CAR T-cell therapy may be present in some patients from the time of upfront therapy.
Chavez: AstraZeneca: Research Funding; Merk: Research Funding; ADC Therapeutics: Consultancy, Research Funding; BMS: Speakers Bureau; MorphoSys, Bayer, Karyopharm, Kite, a Gilead Company, Novartis, Janssen, AbbVie, TeneoBio, and Pfizer: Consultancy; MorphoSys, AstraZeneca, BeiGene, Genentech, Kite, a Gilead Company, and Epizyme: Speakers Bureau. Shah: Pfizer: Consultancy, Other: Expenses; Incyte: Research Funding; Acrotech/Spectrum: Honoraria; BeiGene: Consultancy, Honoraria; Kite, a Gilead Company: Consultancy, Honoraria, Other: Expenses, Research Funding; Pharmacyclics/Janssen: Honoraria, Other: Expenses; Precision Biosciences: Consultancy; Amgen: Consultancy; Novartis: Consultancy, Other: Expenses; Servier Genetics: Other; Jazz Pharmaceuticals: Research Funding; Bristol-Myers Squibb/Celgene: Consultancy, Other: Expenses; Adaptive Biotechnologies: Consultancy. Nishihori: Karyopharm: Research Funding; Novartis: Research Funding. Lazaryan: Kadmon: Consultancy; Avrobio: Membership on an entity's Board of Directors or advisory committees; Humanigen: Membership on an entity's Board of Directors or advisory committees. Davila: Precigen: Research Funding. Locke: Wugen: Consultancy, Other; Umoja: Consultancy, Other; Cowen: Consultancy; EcoR1: Consultancy; Takeda: Consultancy, Other; Novartis: Consultancy, Other, Research Funding; Legend Biotech: Consultancy, Other; Janssen: Consultancy, Other: Scientific Advisory Role; Kite, a Gilead Company: Consultancy, Other: Scientific Advisory Role, Research Funding; Iovance Biotherapeutics: Consultancy, Other: Scientific Advisory Role; GammaDelta Therapeutics: Consultancy, Other: Scientific Advisory Role; Cellular Biomedicine Group: Consultancy, Other: Scientific Advisory Role; Calibr: Consultancy, Other: Scientific Advisory Role; BMS/Celgene: Consultancy, Other: Scientific Advisory Role; Bluebird Bio: Consultancy, Other: Scientific Advisory Role; Amgen: Consultancy, Other: Scientific Advisory Role; Allogene Therapeutics: Consultancy, Other: Scientific Advisory Role, Research Funding; Emerging Therapy Solutions: Consultancy; Gerson Lehrman Group: Consultancy; Moffitt Cancer Center: Patents & Royalties: field of cellular immunotherapy. Gaballa: Adaptive Biotechnologies: Research Funding; Epizyme: Consultancy, Research Funding; TG therapeutics: Consultancy, Speakers Bureau; Beigene: Consultancy; ADC Therapeutics: Consultancy. Jain: Kite/Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; BMS/Celgene: Consultancy, Honoraria; Takeda: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal